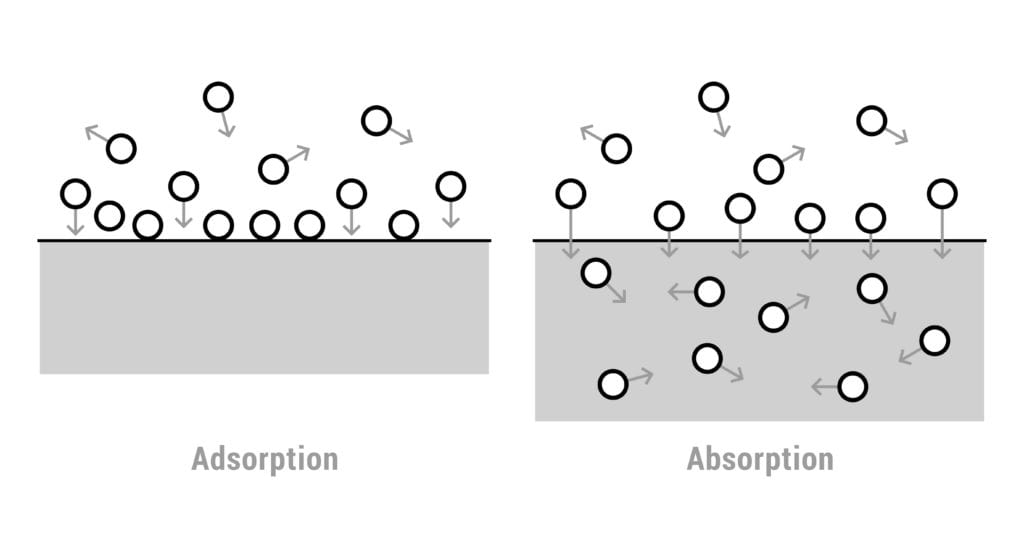
Adsorption occurs when atoms or molecules adhere to the surface of a different material rather than dissolving into it. This process involves surface interaction, where particles form a thin layer on the exterior of solids or liquids.
Adsorption is commonly observed with byproduct atoms and molecules present in:
- Liquids
- Dissolved solids
- Gases
Understanding Adsorption
A simple example of adsorption is wet sand. When sand becomes damp, water molecules bond to the surface of the sand particles. The water does not penetrate the sand itself; instead, it adheres to the surface.
When the damp sand is heated, the water molecules vaporize and separate, demonstrating that the bonding was surface-based rather than absorbed internally.
Adsorption vs Absorption
- Adsorption: Molecules stick to a surface
- Absorption: Molecules dissolve or are taken into the bulk of a material
This is strictly a surface phenomenon, which is why it is widely used in industrial control and recovery systems.
Industrial Applications
Fuel and Petroleum Storage Tanks
One common industrial occurrence of adsorption happens in storage tanks containing petroleum products, such as gasoline, LPG, or natural gas.
During storage:
- Fuel products naturally release vapors
- Vapors form as a byproduct of fuel and air interaction
- Vaporized fuel remains within the storage tank
This vapor formation is unavoidable and occurs due to temperature changes and pressure fluctuations.
Vapor Formation and Product Loss
The vaporization of fuel inside storage tanks leads to:
- Daily product loss due to evaporation
- Increased emissions
- Potential environmental and safety concerns
Without control systems, vaporized hydrocarbons can escape into the atmosphere, resulting in economic loss and regulatory issues.
Benefits of Adsorption in Industrial Operations
- Minimizes product loss
- Reduces emissions
- Enhances safety
- Supports regulatory compliance
- Improves operational efficiency
Adsorption plays a vital role in fuel handling, storage, and vapor control systems.
Adsorption is a process where atoms or molecules adhere to the surface of another material rather than dissolving into it.
Wet sand is a common example water molecules stick to the surface of sand particles and can be removed by heating.
Adsorption systems capture vaporized fuel molecules and convert them back into liquid form, reducing product loss.
Fuel vapors form naturally due to evaporation caused by temperature and pressure changes inside the tank.
They reduce evaporation losses, lower emissions, improve safety, and help meet environmental regulations.



